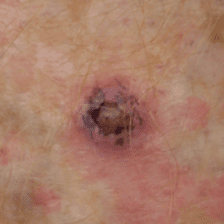
Squamous Cell Carcinoma
What is Squamous Cell Carcinoma?
Squamous cell carcinoma (SCC) is the second most common type of skin cancer
A SCC arises from the skin surface. It typically will arise as an enlarging pink/red lump which may be scaly and often is tender. It generally occurs within areas of pre-existing sun damage and is related to cumulative sun exposure over the years.
Unlike BCCs, SCCs do have the ability to spread to other parts of the body. The risk of this occurring depends on its size, its location and its sub-type. SCCs can grow rapidly and should be dealt with in a timely manner. If neglected they can lead to serious injury including death.
Often SCCs can be clinically diagnosed however a biopsy may be performed to help guide management. Surgical excision of these cancers is often required but your specialist will discuss treatment options with you.